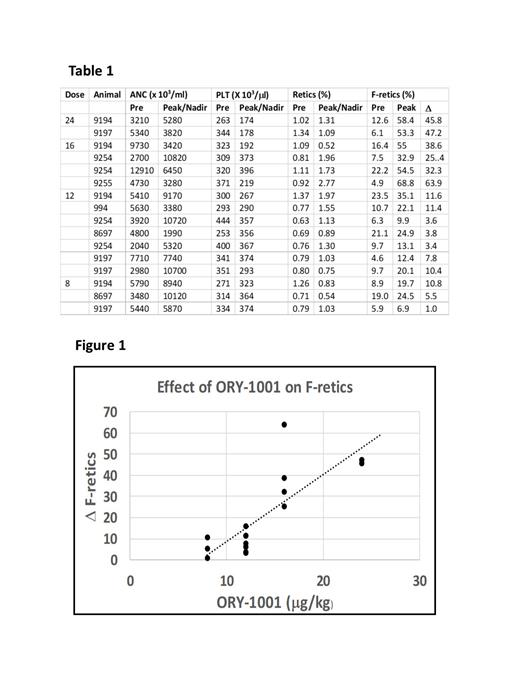
Elevated levels of Fetal Hemoglobin (HbF) reduce the severity of symptoms and increase life span in patients with sickle cell disease (SCD). Experiments performed in baboons ( P. anubis) have been instrumental in discovering the potent pharmocological agents that increase HbF in vivo. Experimental baboon studies have been directly translated to clinical trials in patients with sickle cell disease. Drugs such as DNMT1 and LSD1 inhibitors that attack the repressive epigenetic regulatory mechanisms that silence expression of the γ-globin gene in adult erythroid cells increase HbF in baboons to levels necessary to provide therapeutic relief in SCD patients. Dose-dependent adverse events associated with the use of these drugs are minimized by modifications of dose and schedule as shown by a clinical trial of oral THU-decitabine in 25 SCD patients (Molokie et al, Plos Med 2017;14(9):e10022382). Our recent results have shown that subcutaneous administration of the DNMT1 inhibitor decitabine and the LSD1 inhibitor RN-1 2d/week produced synergistic increases in F-retics, F cells and γ-globin mRNA in normal baboons and increased HbF to therapeutic levels in phlebotomized anemic baboons after administration of only four doses (Ibanez et al, Blood Adv 2023;7(15):3891-3902). The goal of this current study is to develop an oral combinatorial drug regimen using THU-decitabine and the LSD1 inhibitor ORY-1001 that could be directly translated to clinical trials. Our previous studies demonstrated the effectiveness of oral THU-decitabine to increase HbF levels in baboons (Lavelle et al, Blood 2012;119(5):1240-1247). In this study we have performed 16 dose-response experiments in baboons to test the effect of oral ORY-1001 at doses ranging between 8 and 24µg/kg/d. ORY-1001 was chosen because it exhibited a good safety profile with known pharmacokinetic and pharmacodynamic parameters in a recent study in patients with relapsed/refractory acute myeloid leukemia (Salamero et al, J Clin Oncol 2020; 38(36):4260-4273). Four doses were tested (24µg/kg, n=2; 16µg/kg n=4; 12µg/kg, n-=7; 8µg/kg, n=3). The drug was administered on a single day to facilitate further combinatorial studies with THU-decitabine. Preliminary data from a current clinical trial of THU-decitabine in SCD patients (NCT04055818) suggests that administering the drug on a 1d per week schedule effectively increased HbF levels in patients. ORY-1001 was administered by gavage on a single day (d1) and the effect on F-retics measured by flow cytometry in peripheral blood samples obtained daily between days 6 and 10. The peak F-retic response was observed on d8. A linear dose-response relationship on F-retics was observed (Figure 1, r=0.79). Complete blood counts (CBC) were also performed daily between d6 and d10. Adverse hematological effects were not observed with no incidences of neutropenia or thrombocytopenia (Table 1). No consistent effects on reticulocyte counts were observed (Table 1). These results show that a single oral dose of ORY-1001 is sufficient to increase HBF in baboons without any adverse hematological effects and provide the necessary dose-response data to pursue further combinatorial studies with oral THU-decitabine
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal